Multiple choice chemistry questions
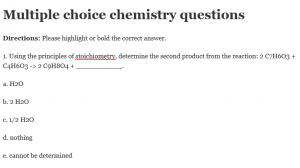
Directions: Please highlight or bold the correct answer.
1. Using the principles of stoichiometry, determine the second product from the reaction: 2 C7H6O3 + C4H6O3 -> 2 C9H8O4 + ___________.
a. H2O
b. 2 H2O
c. 1/2 H2O
d. nothing
e. cannot be determined
2. For the previous reaction, if we begin with 1.10 moles of C7H6O3 and 0.850 moles of C4H6O3, what is the theoretical number of moles of the organic product, C9H8O4?
a. 2.11 moles
b. 2.55 moles
c. 4.00 moles
d. 1.10 moles
e. 0.85 moles
3. If the reaction in 19 yields 0.73 moles of C9H8O4, what is the percent yield (rounded)?
a. 66%
b. 42%
c. 34%
d. 85%
e. 11%
4. How does one calculate a mole?
a. Grams product divided by grams starting material
b. Grams of a material divided by molecular weight of same material
c. 6.023 molecules divided by the temperature
d. Grams of product divided by 6.023 molecules
e. Weight of the sample times the volume
5. What is the value of calculating a mole?
a. It allows someone to equate different materials in terms of numbers of units of each
b. It is a way of determining the amount of product possible from starting reagents
c. It is a method for determining the reaction conditions
d. It tells you how hot the reaction is.
e. It allows someone to equate different materials in terms of numbers of units of each and it is a way of determining the amount of product possible from starting reagents
6. Balancing a chemical equation tells you:
a. how much of each material can be present in a reaction.
b. the limiting reagent.
c. what kind of atoms are present and in what amounts.
d. all of the choices apply.
7. What is the empirical formula of the sugar glucose: C6H12O6?
a. C2H2O2
b. CH2O
c. C6H12O6
d. CH4O
e. H2O
8. Calculate the molecular weight of glucose: C6H12O6.
a. 180.16
b. 180.16g/mol
c. 18.0g/mol
d. 182.36g/mol
e. 184.52g/mol
9. If one wishes to prepare any sugar from the elements, which elements need not be present?
a. Hydrogen
b. Oxygen
c. Carbon
d. Water
e. Bromine
10. A mass spectrometer tells you:
a. the elemental composition of a sample.
b. the empirical formula of a sample.
c. how much energy is in the sample.
d. the elemental composition of a sample and the empirical formula of a sample.
e. the elemental composition of a sample and how much energy is in the sample.
11. The term, empirical formula, is:
a. the number of atoms in a compound.
b. the relative number of atoms in a compound.
c. the number of molecules in a mole.
d. the relative number of compounds in a sample.
e. none of the choices apply.